Abstract
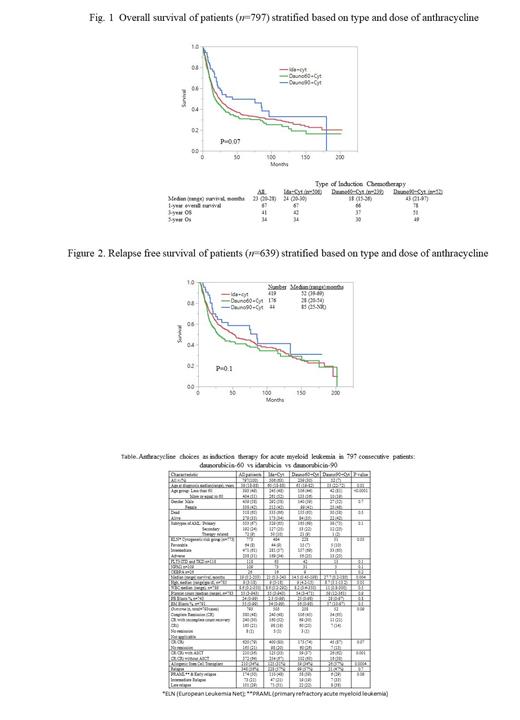
Objective : We describe the Mayo Clinic experience in 797 newly-diagnosed patients with acute myeloid leukemia (AML) serially treated with 7+3 induction chemotherapy that included 3 days of daunorubicin at a daily dose of 60 mg/m2 (dauno-60; n=239) or 90 mg/m2 (dauno-90; n=52), or idarubicin 12 mg/m2 (IDA-12; n=506). Our objective was to compare overall (OS) and relapse-free (RFS) survival outcome.
Methods : Newly-diagnosed AML patients seen at our institution and received intensive induction chemotherapy were identified from the Mayo Clinic AML database. Treatment period spanned from January 2004 through May 2021. Follow-up information was updated as of June 2021. Conventional criteria were used to diagnose AML, assign cytogenetic risk category, and classify treatment response.
Results : The study group included 797 patients (median age 60 years, range 18-88; 58% males): 506 (63%) patients received IDA-12, 239 (30%) dauno-60, and 52 (7%) dauno-90. The respective median (range) ages were 60 (18-88), 61 (19-82), and 53 (22-72) years (p=0.01) (Table). Primary, secondary, and therapy-related AML accounted for 65%, 25% and 10% of patients treated with IDA-12, 69%, 22%, and 9% of those treated with dauno-60, and 75%, 23% and 2% of patients treated with dauno-90, respectively (p=0.1). The corresponding frequencies of adverse karyotype were 34%, 25% and 25% (p=0.05).
CR/CRi was documented in 78% (620/793) of all evaluable patients: IDA-12 80% (400/503), dauno-60 75% (175/238), and dauno-90 87% (45/52) (p=0.1). 210 (34%) patients underwent allogenic hematopoietic stem cell transplant (AHSCT), including 125 (25%), 59 (25%) and 26 (50%) patients, in the three treatment groups, respectively (p=0.0004). After a median (range) follow up 19 (0.2-203) months, 348 (54%) relapses and 518 (65%) deaths were documented.
Median (range) survivals for IDA-12, dauno-60 and dauno-90 groups were 21 (0.3-243), 14.5 (0.45-198), and 27.7 (0.2-180) months (p=0.07; figure 1). The respective 1-, 3-, and 5-year OS rates were 67%, 42%, and 34% (IDA-12); 66%, 37%, and 30% (dauno-60); and 78%, 51%, and 49% (dauno-90), respectively; the trend favoring dauno-90 was no longer apparent during age-adjusted analysis (p=0.33). Multivariable analysis that accounted for age, cytogenetic risk category, FLT3-ITD/NPM1 status and AML subtype confirmed the lack of additional contribution from IDA-12 vs dauno-60 vs dauno-90 (p=0.2) while affirming the independent prognostic value of the other four variables; AHSCT carried an additional predictive value for superior survival without altering these results. A total of 348 (54%) relapses were documented: 228 (57%) in the IDA-12; 99 (57%) in the dauno-60; and 21 (47%) in the dauno-90 cohorts (p=0.7); RFS was similar in the three treatment groups (p=0.1; figure 2).
Conclusion :In the current large single-institution study of consecutive adult patients with AML, neither the choice of anthracycline (idarubicin vs daunorubicin) or the dose of daunorubicin (60 vs 90 mg/m2) appeared to effect outcome in terms of remission rates or overall or relapse-free survival. The study otherwise confirms the independent favorable effect of younger age, non-adverse karyotype, FLT3-ITD-/NPM1+ status, and AHSCT.
Patnaik: Kura Oncology: Research Funding; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees. Al-Kali: Astex: Other: Research support to institution; Novartis: Research Funding. Litzow: Pluristem: Research Funding; Actinium: Research Funding; Amgen: Research Funding; Jazz: Other: Advisory Board; Biosight: Other: Data monitoring committee; Omeros: Other: Advisory Board; AbbVie: Research Funding; Astellas: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal